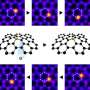
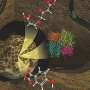
Graphical abstract. Credit: ACS Nano (2024). DOI: 10.1021/acsnano.3c07853
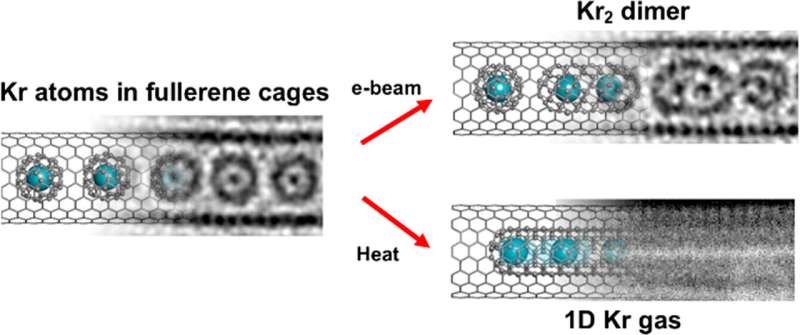
For the first time, scientists have successfully trapped atoms of krypton (Kr), a noble gas, inside a carbon nanotube to form a one-dimensional gas.
Scientists from the University of Nottingham's School of Chemistry used advanced transmission electron microscopy (TEM) methods to capture the moment when Kr atoms joined together, one by one, inside a "nano test tube" container with diameter half a million times smaller than the width of a human hair. The research has been published in ACS Nano.
The behavior of atoms has been studied by scientists ever since it was hypothesized that they are the basic units of the universe. The movement of atoms has significant impact on fundamental phenomena such as temperature, pressure, fluid flow and chemical reactions.
Traditional spectroscopy methods can analyze the movement of large groups of atoms and then use averaged data to explain phenomena at the atomic scale. However, these methods don't show what individual atoms are doing at a specific point in time.
The challenge researchers face when imaging atoms is that they are very small, ranging from 0.1–0.4 nanometers, and they can move at very high speeds of around 400 m/s in the gas phase, on the scale of the speed of sound. This makes the direct imaging of atoms in action very difficult, and the creation of continuous visual representations of atoms in real time remains one of the most significant scientific challenges.
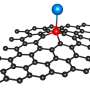
Professor Andrei Khlobystov, School of Chemistry, University of Nottingham, said, "Carbon nanotubes enable us to entrap atoms and accurately position and study them at the single-atom level in real-time. For instance, we successfully trapped noble gas krypton (Kr) atoms in this study. Because Kr has a high atomic number, it is easier to observe in a TEM than lighter elements. This allowed us to track the positions of Kr atoms as moving dots."
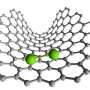
Single atoms of Kr entrapped in C60 fullerene cages within nanotube. Credit: University of Nottingham
Professor Ute Kaiser, former head of the Electron Microscopy of Materials Science group, senior professor at the University of Ulm, added, "We used our state-of-the-art SALVE TEM, which corrects chromatic and spherical aberrations, to observe the process of krypton atoms joining together to form Kr2 pairs."
"These pairs are held together by the van der Waals interaction, which is a mysterious force governing the world of molecules and atoms. This is an exciting innovation, as it allows us to see the van der Waals distance between two atoms in real space. It's a significant development in the field of chemistry and physics that can help us better understand the workings of atoms and molecules."
The researchers utilized Buckminster fullerenes, which are football-shaped molecules consisting of 60 carbon atoms, to transport individual Kr atoms into nano test tubes. The coalescence of buckminsterfullerene molecules to create nested carbon nanotubes helped to improve the precision of the experiments.
Ian Cardillo-Zallo, a Ph.D. student at the University of Nottingham, who was responsible for the preparation and analysis of these materials, said, "Krypton atoms can be released from the fullerene cavities by fusing the carbon cages. This can be achieved by heating at 1,200°C or irradiating with an electron beam. Interatomic bonding between Kr atoms and their dynamic gas-like behavior can both be studied in a single TEM experiment."
The group has been able to directly observe Kr atoms exiting fullerene cages to form a one-dimensional gas. Once freed from their carrier molecules, Kr atoms can only move in one dimension along the nanotube channel due to the extremely narrow space. The atoms in the row of constrained Kr atoms cannot pass each other and are forced to slow down, like vehicles in traffic congestion.
More information: Atomic-Scale Time-Resolved Imaging of Krypton Dimers and Chains and the Transition to a One-Dimensional Gas, ACS Nano (2024). DOI: 10.1021/acsnano.3c07853
Journal information: ACS Nano
Provided by University of Nottingham