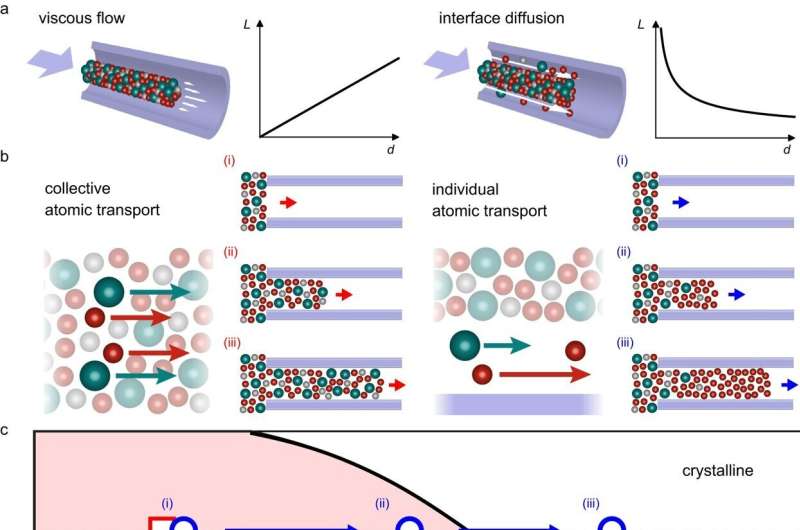
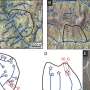
Transition between collective and individual atomic transport. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-41582-2
The matter of how metals deform or respond to external stresses has been extensively studied among metallurgists for centuries. When it comes to conventional metals—the crystalline kind with atoms that line up in neat patterns—the process is fairly well understood. But for the deformation of metallic glasses and other amorphous metals, easy answers have been elusive, particularly when it comes to how things work at the nanoscale.
In a new study, Prof. Jan Schroers looks at the physical quirks of how these metals behave at very small sizes—insights that could lead to new ways of creating metallic glasses. The results are published in Nature Communications.
Materials with the strength of metal but with the pliability of plastic, metallic glasses are being developed for a broad range of applications: aerospace, space, robotics, consumer electronics, sporting goods, and biomedical uses.
These materials owe their properties to their unique atomic structures: when metallic glasses cool from a liquid to a solid, their atoms settle into a random arrangement and do not crystallize the way traditional metals do. But preventing atoms from crystallizing is tricky, and any insights into their workings could go a long way toward more efficient production of metallic glass.
"To advance fabrication and use of amorphous metals, a fundamental and complete understanding of their size- and temperature-dependent deformation is required," the study's authors write.
In the last few decades, it's been well-established that at the macroscopic scale, atoms move en masse when deforming at temperatures that allow flow.
"They deform in a collective way, almost like honey," said Schroers, the Robert Higgin Professor of Mechanical Engineering and Materials Science. "You see all of these atoms kind of moving collectively together."
But what happens when nanoscale-size samples deform? Using zirconium copper and other metallic glass samples in a soft state, the Schroers lab decided to find out.
"Naijia Liu, the grad student in my lab, created smaller and smaller samples, and at some point he could show that they don't deform that way anymore," Schroers said. At sample sizes of 100 nanometers or smaller, things began to veer from the standard rules.
What they found was that at this size, the samples' chemical composition would never change if the atoms continued to move collectively. What happened instead was that the atoms moved individually, and at a certain point, the metal began deforming rapidly.
"So if you go smaller and smaller, then the atoms, they don't flow anymore. What they do instead is travel individually over the surface."
That's significant because atoms are known to move faster on the surface of crystalline materials. So, the smaller the sample, the greater proportion of the material is on, or close to a surface. In order to deform, atoms take an extra distance by using such a fast surface path as it allows general faster deformation. It's an insight into an area of physics that still has many unanswered questions.
"We know essentially everything about crystals, and we know essentially everything about gases," Schroers said. "But in the scientific community, we do not know the liquid state well. Things move around too quickly, so observation methods are challenged and as the order in a liquid is non-periodic, we can't reduce the problem to a smaller unit."
Schroers' lab currently focuses on which alloys are most promising for creating metallic glasses through this method. "The alloy should comprise similar elements, but not too similar, as otherwise the template on which they are growing cannot be formed into a glass," Schroers said.
Besides the scientific impact of their new findings, Schroers said, the study has significance on a technological level. Instead of the current technique of avoiding crystallization through very fast cooling, these findings provide researchers with a novel method to slowly grow metastable materials. These materials include metallic glasses and even others that previously weren't possible to make with other techniques.
More information: Naijia Liu et al, Size-dependent deformation behavior in nanosized amorphous metals suggesting transition from collective to individual atomic transport, Nature Communications (2023). DOI: 10.1038/s41467-023-41582-2
Provided by Yale University