Credit: Nano Letters (2023). DOI: 10.1021/acs.nanolett.3c01537
In chemistry, structure is everything. Compounds with the same chemical formula can have different properties depending on the arrangement of the molecules they're made of. And compounds with a different chemical formula but a similar molecular arrangement can have similar properties.
Graphene and a form of boron nitride called hexagonal boron nitride fall into the latter group. Graphene is made up of carbon atoms. Boron nitride, BN, is composed of boron and nitrogen atoms. While their chemical formulas differ, they have a similar structure—so similar that many chemists call hexagonal boron nitride "white graphene."
Carbon-based graphene has lots of useful properties. It's thin but strong, and it conducts heat and electricity very well, making it ideal for use in electronics.
Similarly, hexagonal boron nitride has a host of properties similar to graphene that could improve biomedical imaging and drug delivery, as well as computers, smartphones and LEDs. Researchers have studied this type of boron nitride for many years.
But, hexagonal boron nitride isn't the only useful form this compound comes in.
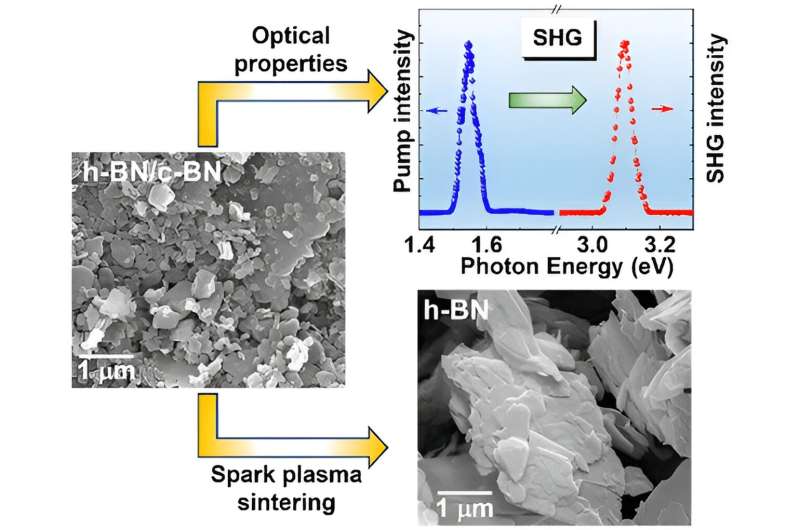
As materials engineers, our research team has been investigating another type of boron nitride called cubic boron nitride. We want to know if combining the properties of hexagonal boron nitride with cubic boron nitride could open the door to even more useful applications.
Hexagonal versus cubic
Hexagonal boron nitride is, as you might guess, boron nitride molecules arranged in the shape of a flat hexagon. It looks honeycomb-shaped, like graphene. Cubic boron nitride has a three-dimensional lattice structure and looks like a diamond at the molecular level.
H-BN is thin, soft and used in cosmetics to give them a silky texture. It doesn't melt or degrade even under extreme heat, which also makes it useful in electronics and other applications. Some scientists predict it could be used to build a radiation shield for spacecraft.
C-BN is hard and resistant. It's used in manufacturing to make cutting tools and drills, and it can keep its sharp edge even at high temperatures. It can also help dissipate heat in electronics.
Even though h-BN and c-BN might seem different, when put together, our research has found they hold even more potential than either on its own.
Both types of boron nitride conduct heat and can provide electrical insulation, but one, h-BN, is soft, and the other, c-BN, is hard. So, we wanted to see if they could be used together to create materials with interesting properties.
For example, combining their different behaviors could make a coating material effective for high temperature structural applications. C-BN could provide strong adhesion to a surface, while h-BN's lubricating properties could resist wear and tear. Both together would keep the material from overheating.
Journal information: Nano Letters
Provided by The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()