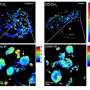
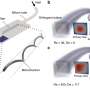
Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abo0049
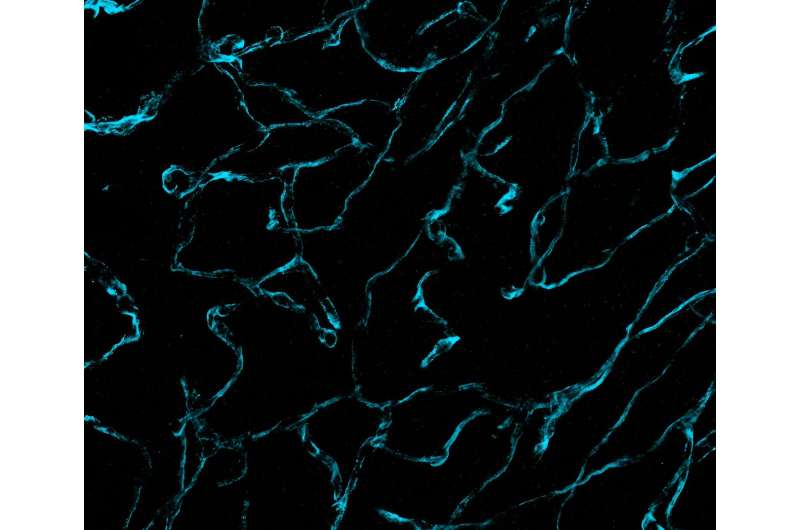
Researchers from Brigham and Women's Hospital and the Massachusetts Institute of Technology (MIT) have unveiled unprecedentedly detailed images of brain cancer tissue through the use of a new microscopy technology called decrowding expansion pathology (dExPath).Their findings, published in Science Translational Medicine, provide novel insights into brain cancer development, with potential implications for advancing the diagnosis and treatment of aggressive neurological diseases.
"In the past, we have relied on expensive, super-resolution microscopes that only very well-funded labs could afford, required specialized training to use, and are often impractical for high-throughput analyses of brain tissues at the molecular level," said Pablo Valdes, MD, Ph.D., a neurosurgery resident alumnus at the Brigham and lead author of the study. "This technology brings reliable, super-resolution imaging to the clinic, enabling scientists to study neurological diseases at a never-before-achieved nanoscale level on conventional clinical samples with conventional microscopes."
Researchers previously relied on costly, super-high-resolution microscopes to image nanoscale structures in cells and brain tissue, and even with the most advanced technology, they often struggled to effectively capture these structures at the nanoscale level.
Ed Boyden, Ph.D., the Y. Eva Tan Professor in Neurotechnology at MIT and co-senior author on this study, began addressing this problem by labeling tissues, and then chemically modifying them to enable uniform physical expansion of tissues. However, this expansion technology was far from perfect. Relying on enzymes known as proteases to break up tissue, scientists found that this chemical treatment with enzymes destroyed proteins before they could analyze them, leaving behind only a skeleton of the original structure, retaining only the labels.
Working together, Boyden and E. Antonio Chiocca, MD, Ph.D., Neurosurgery Chair at Brigham and Women's Hospital and co-senior author on this study, mentored Valdes during his training as a neurosurgeon-scientist, to develop novel chemistries with dExPath to address the limitations of the original expansion technology.
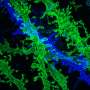
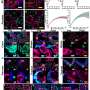
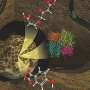
dExPath 3-dimensional image stack of thick, formaldehyde-fixed mouse brain tissue. Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abo0049
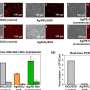
Their new technology chemically modifies tissues by embedding them in a gel and "softening" the tissues with a special chemical treatment that separates protein structures without destroying them and that allows tissues to expand. This provided exciting findings to the MIT and Brigham researchers, who routinely use commercially available antibodies to bind to and illuminate biomarkers in a sample.
Antibodies, however, are large and many times cannot easily penetrate cell structures to reach their target. Now, by pulling proteins apart with dExPath, these same antibodies used for staining can penetrate spaces to bind proteins in tissue that could not be accessed before expansion, highlighting nanometer sized structures or even cell populations that were previously hidden.
"The human brain has several stop guards in place to protect itself from pathogens and environmental toxins. But these elements make studying brain activity challenging. It can be a bit like driving a car through mud and ditches. We cannot access certain cell structures in the brain because of barriers that stand in the way," said E. Antonio Chiocca, MD, Ph.D., chair of the Department of Neurosurgery at the Brigham. "That is just is one of the reasons that this new technology could be so practice changing. If we can take more detailed and accurate images of brain tissue, we can identify more biomarkers and be better equipped to diagnose and treat aggressive brain diseases."
More information: Pablo Valdes et al, Improved immunostaining of nanostructures and cells in human brain specimens through expansion-mediated protein decrowding, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.abo0049
Journal information: Science Translational Medicine
Provided by Brigham and Women’s Hospital