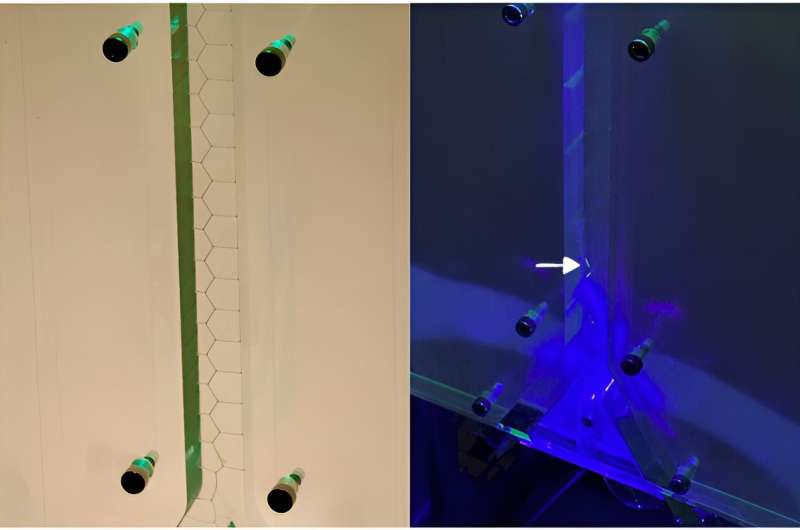
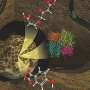
The soap device in operation. On the left: the machine automatically generates a continuous soap film in a zigzag pattern. On the right: fluorescent molecules have been added to the soap film. When exposed to blue light, these molecules emit green light (at the white arrow). Credit: Physical Review Letters (2024). DOI: 10.1103/PhysRevLett.132.028201
A soap film with chemically distinct sides represents the latest breakthrough in research led by chemist Sylvestre Bonnet. This unique soap film, along with an innovative device capable of continuously producing new soap films, forms a crucial piece in the puzzle for the development of artificial photosynthesis. The study is published in Physical Review Letters.
Plants possess a unique ability: Provide them with enough oxygen and water, and with the aid of sunlight, they convert these ingredients into oxygen and sugar, their source of energy. Researchers are exploring ways to mimic this process, aiming to produce sustainable fuel instead of sugar using light-sensitive molecules.
This could be achieved in various ways. However, in the SoFia project, completed in the summer of 2023, Bonnet and his colleagues focused on soap films.
"Here, we mimic the membrane and light-sensitive molecules that a plant uses for photosynthesis," he explains. "Soap is a cheap material, and it requires little energy to create the soap films."
The ultimate goal is to produce carbon monoxide (CO). Bonnet states, "A crucial ingredient for syngas—a mixture of carbon monoxide and hydrogen gas—this gas mixture can then be converted back into liquid fuel, suitable for vehicles."
The team developed a machine that adds light-sensitive molecules to the soap and then produces a continuous flow of uniform soap bubbles. Under the influence of light, and under the right conditions, photosynthesis can eventually occur on the soap films of these bubbles. If successful, the end products of the photochemical reaction are automatically separated at the bottom of the device.
However, there was one problem: Photosynthesis requires a soap film that is chemically different on both sides. "That is a fundamental aspect in nature," explains Bonnet. "When you convert energy into another form of energy, asymmetry is required. The cell membrane of a plant is also asymmetric, as is our skin. On a larger scale, you can think of a motor, where chemical energy is converted into motion. A motor always operates in one direction."
How to customize a soap film without breaking it?
Naturally, the sides of a soap film are chemically identical. The researchers had to find a way to alter the chemistry without breaking the fragile soap film or, in other words, without causing the bubbles to burst.
Bonnet explains, "We had to apply different molecules to both sides, but that was quite challenging. When we tried with a pipette, the bubbles burst. In the end, we used a special mist spray made by colleague Cees van Rijn from the University of Amsterdam. With that spray, we could delicately apply our light-sensitive molecules to the soap film. Spectrometry confirmed that the two sides of the resulting soap film are indeed different."
Now there is a machine that automatically produces soap films and a technique to chemically prepare these films for photosynthesis. "We have also experimented with producing hydrogen through shining blue light on photocatalytic foam," adds Bonnet.
"We now need to combine these three steps. The ultimate goal: a machine that creates asymmetric soap films. That, when you shine light on it, it delivers fuel on one side and oxygen on the other. That is quite revolutionary and ambitious. However, we already have several crucial pieces of the puzzle and are on the right track."
More information: Nidhi Kaul et al, Realizing Symmetry-Breaking Architectures in Soap Films, Physical Review Letters (2024). DOI: 10.1103/PhysRevLett.132.028201
Journal information: Physical Review Letters
Provided by Leiden University