This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
First tetratomic supermolecules realized at nanokelvin temperatures

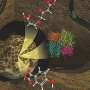
A team of experimentalists at the Max Planck Institute of Quantum Optics (MPQ) and theorists at the Chinese Academy of Sciences (CAS) has succeeded for the first time in populating and stabilizing a new type of molecule, so-called field-linked tetratomic molecules. These "supermolecules" are so fragile that they can only exist at ultracold temperatures. Their existence had long been suspected but has never been demonstrated experimentally—until now.
The polyatomic molecules created in this new study are composed of more than two atoms and have been successfully cooled down to 134 nanokelvin—more than 3,000 times colder than the temperature of previously created tetratomic molecules. This achievement is not only a novel feat in molecular physics, but also a significant step forward in the study of exotic ultracold matter. The research is published in Nature.
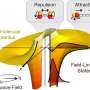
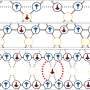
About two decades ago, American theoretical physicist John Bohn and his colleagues predicted a novel type of binding between polar molecules: If the molecules carry an asymmetrically distributed charge—what physicists call polarity—they can combine in an electric field to form weakly bound "supermolecules."
The behavior of these polar molecules can be thought of as compass needles inside a hard shell. When brought close together, compass needles experience an attraction that is stronger than the Earth's magnetic field and they point towards each other instead of aligning north.
A similar phenomenon can be observed with polar molecules, which under specific conditions, can form a unique bound state via electrical forces. Their bond somewhat reminds of a dancing couple holding each other tightly while at the same time constantly maintaining a certain distance.
The supermolecules' bound state is far weaker than typical chemical bonds, but at the same time also much longer reaching. Supermolecules share a bond length over distances that are several hundred times longer than normally bound molecules.
Due to this long-range nature, such supermolecules are highly sensitive: If the parameters of the electric field are changed only a little at a critical value, the forces between the molecules change dramatically—a phenomenon referred to as "field-linked resonance." This enables the researchers to flexibly vary the shape and size of the molecules with a microwave field.
A play in three parts: From diatomic to tetratomic molecules
Ultracold polyatomic molecules contain a rich internal structure that offers exciting new possibilities in cold chemistry, precision measurements, and in quantum information processing. However, their high complexity compared to diatomic molecules poses a major challenge to the employment of conventional cooling techniques such as direct laser cooling and evaporative cooling.
Researchers in the "NaK Lab" (sodium potassium lab) at MPQ, led by Dr. Xin-Yu Luo, Dr. Timon Hilker and Prof. Immanuel Bloch, have achieved a series of pioneering and Nature-published discoveries in recent years, which were crucial to finally overcoming this challenge.
First, in 2021, researchers in this lab invented a novel cooling technique for polar molecules using a high-power rotating microwave field, and thereby set a new low-temperature record: 21 billionths of a degree above absolute zero at minus 273.15 degree Celsius.
A year later, the researchers succeeded in creating the necessary conditions to observe the signature of binding between these molecules in scattering experiments for the first time. This provided the first indirect evidence of the existence of these theoretically long-predicted exotic constructs.
Now, there is even direct evidence as the researchers have been able to create and stabilize these supermolecules in their experiment. Imaging of these "supermolecules" revealed their p-wave symmetry—a unique feature that is crucial in the realization of topological quantum materials, which in turn can be relevant for fault-tolerant quantum computation.
"This research will have immediate and far-reaching implications, " says Xing-Yan Chen, Ph.D. Candidate and first author of the paper. "As the method is applicable to a wide range of molecular species, it allows to explore a much higher variety of ultracold polyatomic molecules. In the future, it could allow to create even bigger and longer-living molecules which would specifically be interesting for precision metrology or quantum chemistry."
"We arrived at these findings thanks also to our close collaboration with Prof. Tao Shi and his team from the CAS," adds Dr. Luo, the principal investigator of the experiment. "Our next goal is to further cool these bosonic 'supermolecules' to form a Bose-Einstein condensate (BEC), where the molecules move together collectively. This prospect holds important potential for our fundamental understanding of quantum physics. What's more amazing is that by simply tuning a microwave field, a BEC of 'supermolecules' can transform into a novel quantum fluid of fermionic molecules preserving the special p-wave symmetry."
More information: Xing-Yan Chen et al, Ultracold field-linked tetratomic molecules, Nature (2024). DOI: 10.1038/s41586-023-06986-6
Journal information: Nature
Provided by Max Planck Society