This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Scientists increase the solubility of an effective antidepressant by a factor of 1,600
The anti-anxiety drug diazepam causes side effects: drowsiness, confusion, and nausea. The same applies to the antidepressant amitriptyline. A possible solution to the problem could be a new compound, GML-3. It simultaneously exhibits the anti-anxiety activity of diazepam and the antidepressant activity of amitriptyline. At the same time, it is devoid of most of their side effects. However, it is not used in pharmaceuticals since it is poorly soluble in water; this is a necessary condition to create convenient dosage forms based on the drug.
Scientists of RUDN University, V.V. Zakusov Research Institute of Pharmacology, and Kurnakov Institute of General and Inorganic Chemistry have found a way to improve its solubility by a factor of 1,600. The study is published in the journal Polymers.
"GML-3 simultaneously exhibits two therapeutic effects that are needed to treat depression. As a rule, patients need to take several strong medications at once, and this can harm the body. Therefore, GML-3, which does not have most of the side effects of diazepam and amitriptyline, could be a promising drug to combat depression. But to create tablets based on GML-3, it is necessary to increase its solubility," said Alexandre Vetcher, Ph.D., Deputy Director of the Nanotechnology Center at RUDN University.
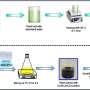
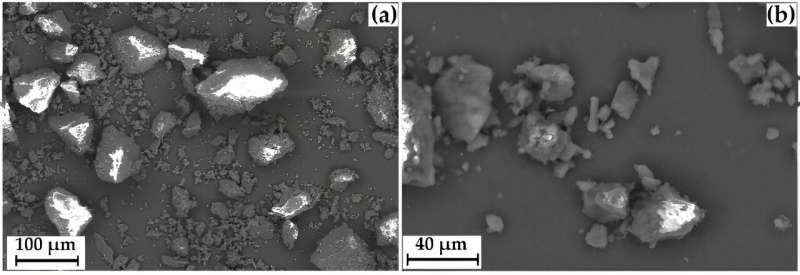
Biochemists have studied several ways to treat GML-3 and find out how they affect solubility. The first way is to crush it with a mortar. The second is to mix it with the water-soluble polymer polyvinylpyrrolidone (PVP). Another approach is the RESS method. The pressure and temperature in the drug solution are increased until GML-3 is completely dissolved, and then quickly sprayed through a narrow nozzle.
Grinding resulted in a fine particle size (about 40 micrometers) but had virtually no effect on solubility. The RESS method made it possible to obtain particles 2,000 times smaller than the original ones—20 to 40 nanometers in size. Solubility increased 430 times.
The addition of PVP removed the residual electrostatic charge on the particles and significantly increased solubility: at a ratio of 1:4 (one part GML-3 to four parts PVP), a solubility of about 80% was achieved within an hour. This is the best result—1,600 times higher than that of conventional GML-3.
"We showed how different grinding methods affect the solubility of GML-3 in water. By itself, it is practically insoluble, the average particle size is about 58.64 micrometers. Mechanical grinding did not affect the dissolution rate," explained Vetcher.
"Moreover, after some time the particles began to stick together and formed agglomerations up to 250 micrometers in size. The best result was shown by the composite that was obtained with a minimum ratio of GML-3 to PVP—one to four. Its solubility has increased 1,600 times."
More information: Vladimir B. Markeev et al, Modeling of the Aqueous Solubility of N-butyl-N-methyl-1-phenylpyrrolo[1,2-a] pyrazine-3-carboxamide: From Micronization to Creation of Amorphous–Crystalline Composites with a Polymer, Polymers (2023). DOI: 10.3390/polym15204136
Provided by Scientific Project Lomonosov