This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New reagent improves process of making sulfur-containing compounds that may be used in medicines
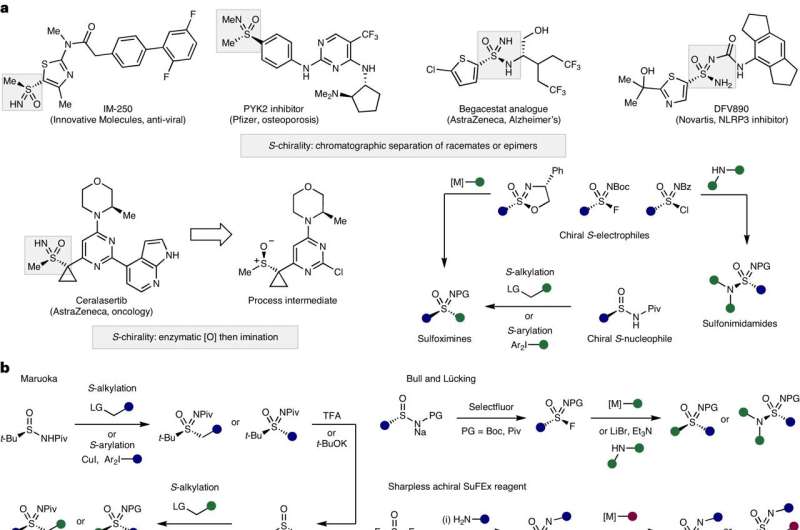
During the past decade, there has been significant development of new sulfur containing compounds that are used in various industries, including pharmaceuticals and agricultural products. Sulfoximines, sulfonimidoyl fluorides and sulfonimidamides are types of sulfur-containing chemical compounds that have wide-ranging potential as therapeutic drugs. However, the synthesis process for these compounds is complex and has several limitations.
In a new article published in Nature Chemistry, Moffitt Cancer Center researchers describe their development of a new reagent that allows a more efficient approach to make sulfoximines, sulfonimidoyl fluorides and sulfonimidamides that may be used in medicines.
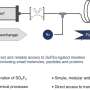
There are four main chemical approaches that are commonly used to create sulfoximines, sulfonimidoyl fluorides and sulfonimidamides. Recently an additional approach called sulfur fluorine exchange (SuFEx) chemistry has gained attention. But the approach has several limitations to its widespread use, such as the requirement for the use of high pressures.
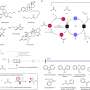
The Moffitt research team wanted to develop a more efficient process to create sulfoximines, sulfonimidoyl fluorides and sulfonimidamides. Through a variety of chemical experiments and processes, they developed a reagent called t-BuSF that serves as a hub in the SuFEx chemical process to synthesize these sulfur-containing compounds. The use of t-BuSF decreased the number of steps required to make these compounds and improved the reaction times and stability of their chemical precursors.
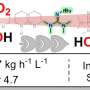
The researchers further demonstrated the potential utility of t-BuSF in medicinal chemistry in over 70 examples and by preparing five therapeutical targets and intermediates. They showed that t-BuSF was able to create these products in high yields with fewer synthesis steps.
"Given the cost-effectiveness and the chemical space accessible from this reagent platform, it is expected to have positive impacts on the discovery sciences from the development of new medicines and agrochemicals to the discovery of new ligands, organocatalysts and materials," said Justin Lopchuk, Ph.D., associate member of the Department of Drug Discovery at Moffitt.
More information: Shun Teng et al, Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent, Nature Chemistry (2024). DOI: 10.1038/s41557-023-01419-3