This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
The effect of additives on calcium carbonate crystallization
Results of a large-scale innovative Citizen Science experiment called Project M, which involved more than 1,000 scientists, 800 samples and 110 U.K. secondary schools in a huge experiment, were published in the journal CrystEngComm.
The paper is titled "Project M: Investigating the effect of additives on calcium carbonate crystallization through a school citizen science program." The paper shares a giant set of results from the school citizen scientists who collaborated with a team at Diamond to find out how different additives affect the different forms of calcium carbonate produced.
These additives affect the type of calcium carbonate that forms, and thus its properties and potential applications. Being able to easily produce different forms of calcium carbonate could be very important for manufacturing.
Lead authors Claire Murray, Visiting Scientist at Diamond and Julia Parker, Diamond Principal Beamline Scientist and expert in calcium carbonate science who conceptualized the project, analyzed the data, and wrote and edited the manuscript explain that despite nature's ability to precisely control calcium carbonate formation in shells and skeletons, laboratories around the world are often unable to exact the same level of control over how calcium carbonate forms.
"Nature uses molecules like amino acids and proteins to direct the formation of calcium carbonate, so we were interested in discovering how some of these molecules affect the calcium carbonate that we make in the lab," said the researchers.
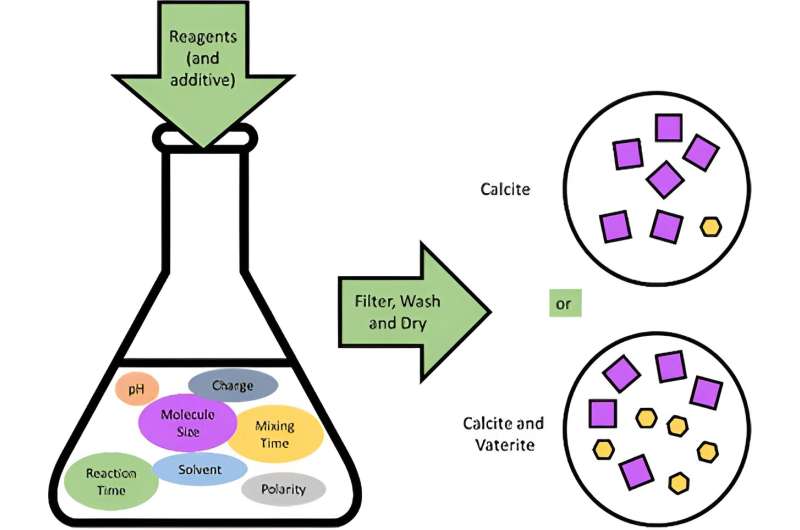
Project M engaged the students and teachers as scientists, making different samples of calcium carbonate under varying conditions with different additives. In all, 800 of these samples were then analyzed in just 24 hours in April 2017 using the X-ray powder diffraction technique at on beamline I11 at Diamond Light Source, the U.K.'s national synchrotron. This created a giant set of results that form the basis of the publication. A systematic study of this scale has never been completed anywhere else in the world.
The goal of this project was to find out how using different additives like amino acids affect the structure of the calcium carbonate. The mineral has three main forms called "polymorphs"—vaterite, calcite and aragonite—which can be identified using X-ray powder diffraction at Diamond's beamline l11.
Diamond Light Source produces one of the brightest X-ray beams on planet Earth, which allow scientists to understand the atomic structure of materials. Scientists come from all over the U.K. and further afield to use these X-rays—as well as infrared and ultraviolet light—to make better drugs, understand the natural world, and create futuristic materials.
Understanding the impact of different additives on the production of polymorphs is of huge interest in industry such as in manufacturing, medical applications such as tissue engineering and the design of drug-delivery systems, and even cosmetics.
However, mapping such a large parameter space, in terms of additive and concentration, requires the synthesis of a large number of samples and the provision of high throughput analysis techniques. It presented an exciting opportunity to collaborate with 110 secondary schools making real samples to showcase the high-throughput capability of the beamline, including rapid robotic changing of samples, which means diffraction patterns can be collected and samples changed in less than 90 seconds.
"The project was led by a scientific question we had," explained Murray. "The idea to involve school students and teaching staff in the preparation of the samples followed naturally as we know chemistry projects are underrepresented in the citizen science space. The contribution that student citizen scientists can make to research should not be underestimated. These projects can provide a powerful way for researchers to access volumes of data they might struggle to collect otherwise, as well as inspiring future generations of scientists."
The project was designed with kit and resources to support the schools to learn new techniques and knowledge and to provide them with space to interact and engage with the experiment. After analysis at Diamond, the students had the opportunity to look at their results, see their peaks and determine what sort of polymorphs they had produced, and compare their results with the results obtained by different samples and different schools at different locations in the U.K.
Gry E. Christensen, former student and Project M Scientist at Didcot Girls' School, Didcot commented, "It was an amazing journey and I recommend that if any other schools have a chance to help with a similar project, then jump on board, because it is a once in a lifetime opportunity for the students, and you feel you can make a positive change to the world."
"The fact that we didn't know the answer yet was a motivational factor for the students," explains Murray. "The teachers told us they took everything more seriously, because this was real science in action—it really meant something. They shared how the students were excited to translate their lab skills to this experiment and that the students were able to contextualize their learning from their prescribed textbooks and lab classes.
"Teachers also highlighted their own interest and curiosity as many of them have trained as chemists in their education. They appreciated the connection to real science for themselves and the opportunity for continued professional development."
Matthew Wainwright, teacher and Project M Scientist at Kettlethorpe High School, Wakefield, adds, "The project offered our pupils a unique opportunity to take part in genuine scientific research and should act as a blueprint for future projects that aim to engage young people in science beyond the classroom."
Exploring the role of amino acids in directing crystallization with the Project M Scientists was an opportunity and an honor for the authors. Parker explained, "In our work we see how we can draw novel scientific conclusions regarding the effect of amino acids on the structure of calcite and vaterite calcium carbonate polymorphs.
"This ability to explore a wide parameter space in sample conditions, while providing continued educational and scientific engagement benefits for the students and teachers involved, can we hope in future be applied to other materials synthesis investigations."
Project M enabled schools to carry out real research and do an experiment that had never been done before, in their own school laboratory. It was the first "citizen science" project run by Diamond, which transported Diamond science to schools and enabled the production of a considerable set of results, which has now resulted in this successful publication in CrystEngComm.
More information: Claire A. Murray et al, Project M: investigating the effect of additives on calcium carbonate crystallisation through a school citizen science program, CrystEngComm (2024). DOI: 10.1039/D3CE01173A
Provided by Diamond Light Source