This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Selective conversion of CO₂ into dimethyl ether over hydrophobic and gallium-modified copper catalysts

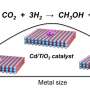
The selective conversion of CO2 and H2 into valuable chemicals and fuels is a promising route for carbon recycling. Multiple routes have been developed for the CO2 hydrogenation to methanol, higher alcohols, dimethyl ether (DME), aromatics, hydrocarbon, and olefins. Among these products, DME is attractive because it is nontoxic and noncorrosive and has been used as a platform chemical in industry, a carrier for hydrogen, and an additive for fuels.
A series of catalysts has been synthesized for the direct hydrogenation of CO2-to-DME via cascade catalysis involving methanol synthesis and methanol condensation to DME over a supported copper catalyst. However, high DME selectivity was only achieved at low conversion of CO2, resulting in poor one-pass productivity.
When the CO2 conversion increased, abundant by-products of CO, methanol, and hydrocarbons were produced. A recent trend is CO2 to DME conversion over bifunctional catalysts, such as acid oxide-supported copper nanoparticles, but their performance is still unsatisfactory. In addition, the copper nanoparticles were sintered during catalysis, resulting in poor durability.
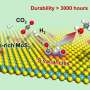
Recently, a research team led by Prof. Feng-Shou Xiao and Prof. Liang Wang from Zhejiang University, China, has overcome these limitations by developing a highly active, selective, and durable copper nanoparticle catalyst for converting CO2 to DME. This was achieved by loading Cu nanoparticles onto hydrophobic and Ga-modified silica supports. The Ga-modified silica provided moderate acidity for methanol dehydration to DME, which hindered deep dehydration to hydrocarbons.
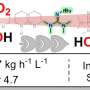
Importantly, the hydrophobic catalyst surface efficiently hinders the sintering of the Cu nanoparticles, which is usually triggered by water and methanol. Consequently, under the following reaction conditions (6000 mL gcat–1·h–1, 3 MPa, 240 °C), the CO2 conversion of 9.7%, DME and methanol selectivities of 59.3% and 28.4%, and CO selectivity of only 11.3% were obtained. In a continuous evaluation for 100 h, the performance was well maintained without any deactivation trend, outperforming the general supported Cu catalysts.
The research is published in the Chinese Journal of Catalysis.
More information: Hangjie Li et al, Selective hydrogenation of CO2 into dimethyl ether over hydrophobic and gallium-modified copper catalysts, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64535-8
Provided by Chinese Academy of Sciences