This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New study explores amino acid that turns into gel in water
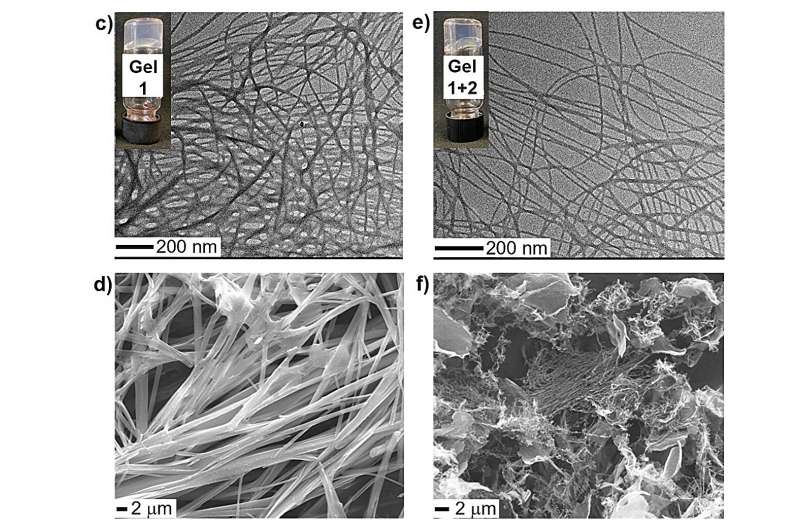
Hydrogels, ubiquitous materials in our daily lives, are the focus of scientific research published in Chemistry—A European Journal. Conducted by the SupraBioNanoLab at the Department of Chemistry, Materials and Chemical Engineering Giulio Natta at Politecnico di Milano, the work demonstrated how the combination of supramolecular chemistry and crystallography can be used to design hydrogels with specific characteristics.
The study focused on the use of an amino acid called Fmoc-pentafluoro-phenylalanine, which effectively turns into a gel in water. The researchers examined the behavior of this molecule in the presence of other substances, including bioactive molecules such as vitamin B3, which establish strong attractive interactions with its reactive groups. Experimental results have shown that the interactions between the amino acid and partner molecules are identical both in the formation of a crystalline complex in the solid state and in the creation of a gel in an aqueous solvent.
"The key to the research was the determination of the crystal structure of the complex through X-ray diffraction, which allowed us to predict the properties and consistency of the resulting gel," explains Valentina Dichiarante of the Department of Chemistry, Materials and Chemical Engineering Giulio Natta at Politecnico di Milano. "This also allowed us to modulate the release of the partner molecule from the gel itself."
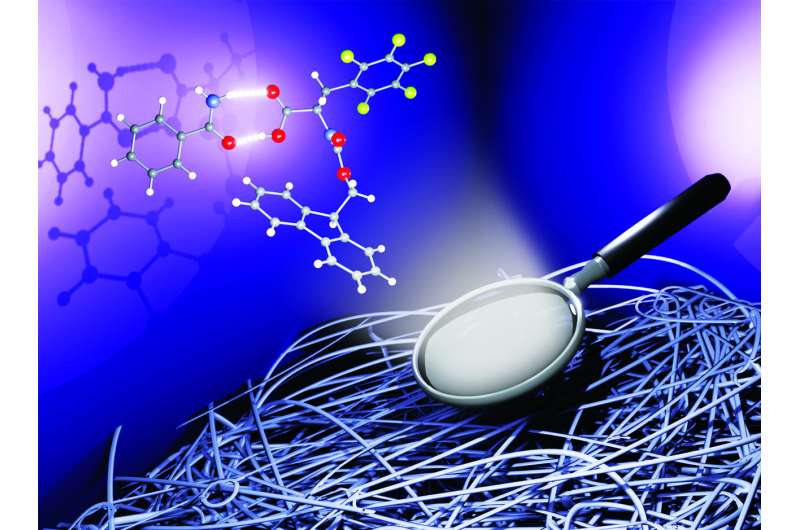
"This scientific breakthrough opens up new perspectives for the selective and targeted design of mixed hydrogels," adds Pierangelo Metrangolo of the Department of Chemistry, Materials and Chemical Engineering Giulio Natta at Politecnico di Milano. "The supramolecular interactions between the solid-phase components allow the strength and structure of the gel to be modulated, creating an ideal matrix for the controlled release of active substances, with possible therapeutic or cosmetic applications."
These results led the journal Chemistry—A European Journal to dedicate the main cover of the edition containing the article to it, together with a detailed profile on the authors of the work and their research activity. This award underlines the significant contribution this research brings to the emerging field of hydrogels and biomedical applications.
More information: Eleonora Veronese et al, Acid⋅⋅⋅Amide Supramolecular Synthon for Tuning Amino Acid‐Based Hydrogels' Properties, Chemistry—A European Journal (2023). DOI: 10.1002/chem.202301743
Journal information: Chemistry – A European Journal
Provided by Polytechnic University of Milan