This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Direct electroconversion of air to nitric acid realized under mild conditions
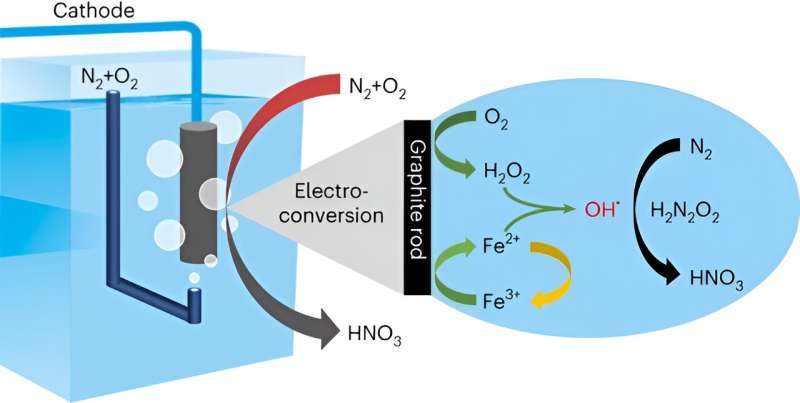
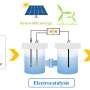
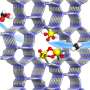
A research group led by Prof. Deng Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has realized direct conversion of air to (nitric acid) HNO3 under ambient conditions through a hydroxyl radical (·OH)-mediated hetero-homogeneous electro-chemical route.
In the route, the N2 was oxidized to HNO3 by employing heterogeneous electroreduction of O2 to H2O2 on a graphite rod catalyst combined with homogeneous Fe2+-induced dissociation of the in situ generated H2O2 to OH· as reactive oxidation species.
The study was published in Nature Synthesis on Sept. 25.
HNO3, as an important chemical building block, is produced industrially from the oxidation of ammonia via the Ostwald process, which requires high temperatures above 800°C. In this process, the feed ammonia gas is industrially synthesized via the energy-intensive Haber-Bosch method typically operating at high temperatures (400 ~ 500°C) and pressures (20 ~ 50 MPa).
Direct conversion of dinitrogen (N2) and oxygen (O2) in the air to HNO3 under mild conditions is a promising and sustainable route for the production of HNO3. However, the thermodynamic and kinetic restrictions of this reaction, as well as the high stability of N2 and the low activity of O2, make it challenging to achieve co-activation and efficient conversion of the two molecules in the air toward HNO3 synthesis.
In this study, the researchers developed a hetero-homogeneous electro-chemical route for the direct conversion of air to HNO3 at the cathode compartment. At the cathodic potential of 0 V vs. RHE, the faradaic efficiency of nitric acid was as high as 25.37%, and the selectivity was over 99%, which was superior than the previously reported electrocatalytic oxidation of N2 at the anode with high potentials above 1.23 V vs. RHE.
Multiple in-situ spectroscopic characterizations and theoretical calculations revealed that ·OH produced from Fe2+-induced dissociation of in-situ generated H2O2 could efficiently activate N2. This delivers a high HNO3 productivity of 141.83 μmol·h-1·gFe-1, which is 225 times that of directly using H2O2 as the oxidant.
"This process for HNO3 synthesis from N2 and O2 effectively avoids the traditional high-temperature and high-pressure reaction conditions, and provides a new route for the co-activation and efficient conversion of N2 and O2 under mild conditions," said Prof. Deng.
More information: Shiming Chen et al, Direct electroconversion of air to nitric acid under mild conditions, Nature Synthesis (2023). DOI: 10.1038/s44160-023-00399-z
Journal information: Nature Synthesis
Provided by Chinese Academy of Sciences