This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biochemists focus on degrading key cancer-driving protein as a potential approach to stop cancer growth
Case Western Reserve University biochemical researchers have identified a new function of a key protein that leads to cancer—a finding they believe could lead to more effective treatments for a range of cancers and other diseases.
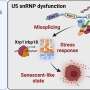
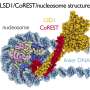
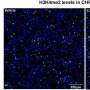
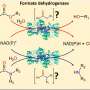
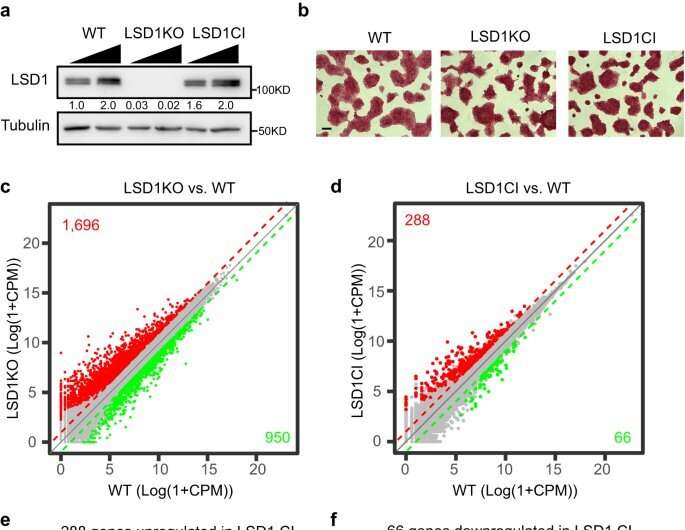
The protein is LSD1 (lysine-specific histone demethylase 1A), which functions as a type of traffic cop inside human cells. It controls gene activity during embryonic development and regulating gene expression throughout life.
Scientists have also identified in recent years that the overexpression of LSD1—in this instance, producing too many proteins—can drive development of cancer and heart disease.
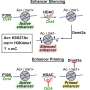
And some researchers have recently looked to slow cancer growth by stopping the catalytic activity of LSDI—the chemical reaction that spurs cell growth, but also appears to lead to its overexpression.
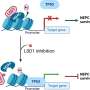
But Kaixiang Cao, an assistant professor of biochemistry, is leading a team that challenges that assumption: The medical school researchers argue that they can achieve far greater success to slow or stop cancer growth in stem cells by instead degrading the entire LSD1 protein, not merely short-circuiting the chemical reaction that leads to its overexpression.
"Our findings really challenge the current paradigm," Cao said.
Their research was published in August in Nature Communications.
"We need a really precise and effective way of targeting these proteins, and our research shows that stopping that catalysis might be effective (at stopping the overexpression) 15% of the time, while our approach is closer to 80%," Cao said. "So, if we can develop a degrader of LSD1, we can help the patient go through less therapy—even if we cannot completely cure cancer."
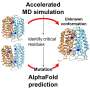
Cao said he and his team were surprised LSD1 functions mainly in a catalytic-independent manner, but now that they've provided to the research community a "theoretical foundation that this is going to be a more effective way to treat these diseases," they'll begin to test further, first in cancerous tissues, then animal models and eventually human trials.
"This is the future—you add the degrader, and it will kill the protein completely," he said. "The technique is already there because it has been done to other proteins by other researchers—but not yet to LSD1."
More information: Cheng Zeng et al, Demethylase-independent roles of LSD1 in regulating enhancers and cell fate transition, Nature Communications (2023). DOI: 10.1038/s41467-023-40606-1
Journal information: Nature Communications
Provided by Case Western Reserve University