This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Carbon-based catalysts for oxygen reduction reaction: Mechanistic understanding and porous structure

Proton exchange membrane fuel cells (PEMFC) have attracted much attention because of high conversion efficiency, low environmental pollution, and high specific energy, which can be widely used in vehicles such as automobiles, airplanes, and stationary power stations.
The cathodic and anodic electrochemical catalysis processes in membrane electrode assembly (MEA) are mainly composed of unit steps such as liquid-phase mass transfer, species adsorption and desorption, electron transfer, and surface transformation occurring at the electrode interface in series.
It implies that electrochemical catalysis is a complex system including multiple scales in space and time: electron transfer at the quantum scale; active sites at the atomic scale; the triple phase boundary and the electrocatalytic ORR mechanism at the molecular level; catalyst and proton conductor at the nanometer scale; the catalytic layer and MEA at the micrometer scale; and the fuel cell stack at the meter scale.
Particularly, each of the scales could greatly affect the electrochemical ORR process and the cell performance. Thus deeply understanding the characteristics and behavior under any scale is the key to greatly enhance the electrocatalytic performance. Of the scales, the catalyst design at the nanometer scale has attracted great attention.
Recently, the research group led by Prof. Zidong Wei and Jing Li from Chongqing University, China, outlined the recent developments of carbon-based ORR catalysts from mechanistic understanding and porous structure. The review was published in Chinese Journal of Catalysis.
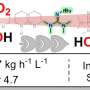
Developing carbon-based catalysts contributes to the large-scale application of fuel cells and metal-air batteries on account of their extremely high cost effectiveness. This review summarized the research progress regarding carbon-based catalysts in terms of the fabrication of active sites, catalyst stability, and strategies for forming porous structures.
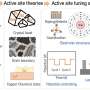
Atomic-scale dispersion and doping are the most used methods in generating active sites in metal-containing carbon-based catalysts. Heteroatomic doping is a common strategy for fabricating active sites in metal-free carbon-based catalysts. We then analyzed the causes of catalyst deactivation and how to improve the stabilities and anti-poisoning properties of the catalysts.
Finally, as the porous structures significantly affect the exposure of the active sites and mass transfer, the effects of the different porous structures on the ORR and the preparation strategies of micro-, meso-, and macroporous carbon materials were reviewed.
More information: Wenjing Zhang et al, Carbon-based catalysts of the oxygen reduction reaction: Mechanistic understanding and porous structures, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64427-4
Provided by Chinese Academy of Sciences