This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers explore the effects of acid hydrolysis on sulfated fucans in sea cucumbers and sea urchins
Cultures from across the globe have used plant and animal extracts as food and traditional medicine. For instance, Asians, especially in Korea, China, and Japan, have used sea cucumber extracts to treat arthritis, frequent urination, impotence, and even cancer. While it is easy to be dismissive of these traditional medicines, sea cucumbers and, in fact, several other marine invertebrates may hold the key to new medicine.
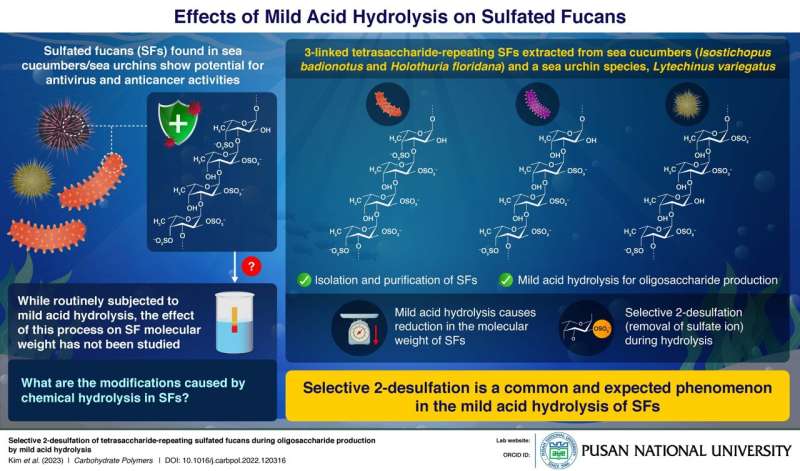
A class of compounds called "sulfated fucans" (SFs), essentially fucose-rich sulfated polysaccharides found in sea cucumbers and sea urchins, are renowned for their anticoagulant, antiviral, and anticancer properties. Recently, they have been investigated for their potency against the SARS-CoV-2 virus.
To study these SFs, one needs to reduce their molecular weight by breaking them down into oligosaccharides. This is often done using a process called "mild acid hydrolysis." Therefore, it is important to know the structural modifications caused by this mild acid hydrolysis on SFs.
This is where a team of researchers led by Professor Seon Beom Kim from Pusan National University in Korea and Assistant Professor Vitor H. Pomin from the University of Mississippi, U.S. came in. In a recent study published in Carbohydrate Polymers, they studied the mild acid hydrolysis of SFs extracted from two sea cucumber species, Isostichopus badionotus and Holothuria floridana, and one sea urchin species, Lytechinus variegatus, to see the effects of this process during oligosaccharide production.
The study involved contributions from Dr. Marwa Farrag, Dr. Sushil K. Mishra, Dr. Sandeep K. Mishra, Dr. Joshua S. Sharp, and Dr. Robert J. Doerksen, all of them collaborating with Dr. Pomin's group.
Speaking about the motivation behind their study, Prof. Kim explains, "One of the keys to being an antiviral agent without showing other biological activities is controlling the molecular weight of the polysaccharide. However, specific enzymes that can depolymerize marine polysaccharides are not widely known. As a result, the mild acid hydrolysis route is often the way to go. Therefore, there is an urgent need for physiochemical studies of SFs during the chemical hydrolysis process."
Following the extraction of the SFs, the team characterized the structure of each of these SFs, revealing that they take the form of long chains of repeating blocks of four sugars containing sulfate (SO42-) ions. Thus, they were classified as 3-linked tetrasaccharide-repeating SFs. Next, these SFs were subjected to mild sulfuric acid and the oligosaccharide produced was investigated to see the changes caused by the hydrolysis.
The researchers found that all three SFs showed a selective 2-desulfation in which the second sugar in the repeating tetrasaccharide lost the sulfate ion attached to it. This caused the long chains to break up and produce an 8-sugar-long oligosaccharide.
"The phenomenon of acid hydrolysis is constantly emphasized for the depolymerization of sulfated fucans. Our study shows that selective 2-desulfation is a common and expected phenomenon in oligosaccharide production by mild acid hydrolysis of SFs," says Prof. Kim. "These results will help further the research on the medicinal properties of SFs, and could potentially result in new medicines for a wide variety of illnesses."
More information: Seon Beom Kim et al, Selective 2-desulfation of tetrasaccharide-repeating sulfated fucans during oligosaccharide production by mild acid hydrolysis, Carbohydrate Polymers (2022). DOI: 10.1016/j.carbpol.2022.120316
Provided by Pusan National University