Evidence that energy metabolism and biofilm formation regulation are intertwined in bacteria
In a recently published article in the journal Trends in Microbiology, author Alberto J. Martin-Rodriguez, Senior Research Specialist at the Department of Microbiology, Tumor and Cell Biology at Karolinska Institutet, explains how he found that distinct bacterial strains selectively use respiration for surface colonization.
Bacteria can live either as single cells- planktonic- or forming multicellular communities, called biofilms. In many ecosystems, this multicellular mode of life is the preferred lifestyle, and it is nowadays clear that many clinically-relevant infections are caused by biofilm bacteria. In biofilms, bacteria can cope better with environmental stressors and are more resistant to the action of antibiotics or the immune system. By forming biofilms, bacteria can also occupy certain niches inside or outside a living host, displacing competitor microbes including the host microbiota.
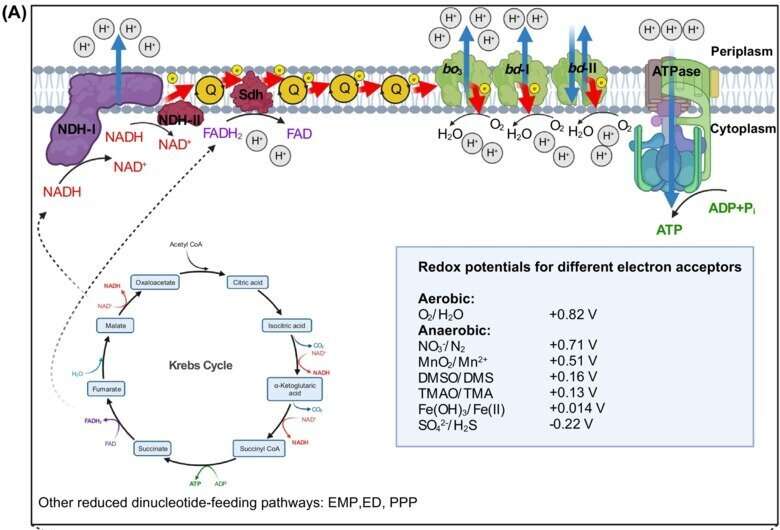
Bacteria living in biofilms are more resistant to antibiotic treatment. The mechanisms linking energy metabolism with biofilm formation may therefore become a target for innovative therapies against recalcitrant biofilm infections, as well as to prevent biofilm formation on biomedical materials. The efficacy of antibiotics has been shown to be intimately linked with bacterial respiration and in this sense, bacterial energy metabolism has already emerged as a target against antimicrobial resistance.
"In this commissioned opinion article, following a series of papers I have led over the last few years, I provide evidence supporting that energy metabolism and regulation of biofilm formation are intimately linked in bacteria, and most importantly, that these links are strain-specific, not limited to energy acquisition per se, and can direct subgroups of strains to occupy certain environmental or host-associated niches," says Alberto J. Martin-Rodriguez. "These findings have important implications in bacterial physiology, niche partitioning, inter-microbial interactions, and bacterial evolution itself."
More information: Alberto J. Martín-Rodríguez, Respiration-induced biofilm formation as a driver for bacterial niche colonization, Trends in Microbiology (2022). DOI: 10.1016/j.tim.2022.08.007
Provided by Karolinska Institutet