Plants can measure the intensity of salt stress

Unfavorable environmental conditions represent considerable stress for plants. A high level of salt content (sodium chloride, NaCl) in the soil is just such a stressor that has a negative impact on plants. Salinization is a serious problem in agriculture especially in dry regions of the world. Biologists at the University of Münster have now discovered, for the first time, that salt stress triggers calcium signals in a special group of cells in plant roots, and that these signals form a "sodium-sensing niche." Also, the researchers identified a calcium-binding protein (CBL8) that contributes to salt tolerance specifically under severe salt stress conditions. The results of the study have now been published in the journal Developmental Cell.
Salt stress is caused by the accumulation of excessive salt concentrations in the soil. This inhibits plant growth and can ultimately lead to the plant dying. For this reason, plant researchers are interested in gaining a better understanding of salt stress in order to breed salt-tolerant plants. Prof. Jörg Kudla and his team at the Institute of Biology and the Biotechnology of Plants at Münster University studied the question of how plants measure the intensity of salt stress and how they react to it. The model plant they used for their tests was thale cress (Arabidopsis thaliana), which is a member of the largest group of flowering plants—the crucifers, or Brassicaceae. These include many food and forage plants such as cabbages, mustard and radishes.
"First of all," says Jörg Kudla, "we examined Arabidopsis roots to see whether they had any type of cells which would react especially to salt stress, or whether the entire root would show a uniform reaction. We also undertook investigations to see whether the intensity of the salt stress was reflected quantitively in the intensity of the calcium signal."
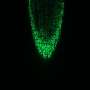
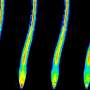
The result surprised the experts: Although the plant's entire root system was exposed to stress, only a specific group of cells reacted—and only this group formed a so-called oligo-cellular calcium signal. This group of cells is located in the differentiation zone of the plant root and is formed by only a few hundred cells. Just for comparison: a root has many thousands of cells. Researchers call this area the "sodium-sensing niche."
"This group of cells," Kudla explains, "is not visible, and we can only distinguish them functionally from other cells by means of high-resolution biosensor technology. It was a chance discovery which was extremely revealing—and significant." The reason is that it is in these functionally specialized cells that the primary calcium signal is formed. In the process, the plant biologists found that the greater the level of salt stress, the stronger the calcium signal.
In other words, the plant is able to provide information to the organism on the intensity of the encountered stress. This led to the question of how plant cells can distinguish between weak and strong calcium signals in order to be able to react accordingly. Generally, calcium signals are decoded by various calcium-binding proteins which act as calcium sensors.
CBL proteins important for salt tolerance
In plants, this important task is often performed by the so-called CBL (calcineurin B-like) proteins. It has been known for some time that the CBL4 protein is important for salt tolerance, and that corresponding mutants without any functioning CBL4 protein are extremely sensitive to salt stress. In their work, the researchers discovered that mutants of a further CBL protein—CBL8—also have reduced salt tolerance. However, cbl8 mutants—in contrast to cbl4 mutants—displayed growth inhibition only under severe salt stress. After carrying out biochemical analyses, the researchers found that a high calcium concentration activates the CBL8 protein—while the CBL4 protein is also active at lower concentrations of calcium. "It is only under conditions of high salt stress that CBL8 helps to pump salt out of the plant," explains Dr. Leonie Steinhorst, who was also involved in the study. "It is a kind of switch mechanism controlled by the concentration of calcium."
One interesting aspect that the biologists discovered in this connection is the evolution of the CBL proteins. Most types of cereals—such as corn, wheat and barley—are so-called monocotyledons. They only have the CBL4 protein—in other words, they lack this switch mechanism for adapting to severe salt stress. There are also dicotyledons, such as tobacco and tomatoes, and it was possible to demonstrate in this case that gene duplication took place early in the evolutionary process and that CBL8 developed from this. As a result, these plants had a better opportunity to react to salt stress.
"So one interesting approach," says Jörg Kudla, "would be to introduce the CBL8 protein into monocotyledons so that they too can better adapt to salt stress. This is likely to be an increasingly important measure for plant breeders in the future in order to cope better with drought and salt stress."
High-resolution microscopy, using molecular calcium biosensor technology in plants, made it possible to discover the oligo-cellular calcium signals already described. These biosensors visualize changes in concentrations of bioactive substances like calcium in cells and tissues. These studies involving in vivo biosensor technology were combined with other genetic, cell-biological and biochemical methods to elucidate in detail the underlying mechanisms.
More information: Leonie Steinhorst et al, A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis, Developmental Cell (2022). DOI: 10.1016/j.devcel.2022.08.001
Journal information: Developmental Cell
Provided by Westfälische Wilhelms-Universität Münster