Transforming sulphur dioxide from harmful to useful

Scientists have created molecular cages within a polymer to trap harmful sulphur dioxide pollution in order to transform it into useful compounds and reduce waste and emissions.
A unique new material developed by an international collaboration of scientists has proved that it can help reduce sulphur dioxide (SO2) emissions in the environment by selectively catching the molecules in minutely engineered cages. The captured toxic gas can then be safely released for conversion into useful industrial products and processes.
Around 87% of sulphur dioxide emissions are the result of human activity, typically produced by power plants, other industrial facilities, trains, ships, and heavy equipment, and can be harmful to human health and the environment. The international team developed porous, cage-like, stable copper-containing molecules known as molecular organic frameworks (MOFs) that are designed to separate sulphur dioxide (SO2) gas from other gases more efficiently than existing systems.
Professor Martin Schröder, Vice-President and Dean of the Faculty of Science and Engineering at the University of Manchester, and Dr. Sihai Yang, a Senior Lecturer in Department of Chemistry at the University of Manchester, led an international research team from UK and U.S. on this work.
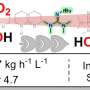
The researchers exposed the MOFs to simulated exhaust gases and found that they efficiently separated out SO2 from the gas mixture at elevated temperatures even in the presence of water.
The research, led by The University of Manchester and published in journal Nature Materials, showed a vast improvement in efficiency compared to current SO2 capture systems, which can produce a lot of solid and liquid waste and may only remove up to 95 percent of the toxic gas, researchers noted.
Conducting state-of-the-art structural, dynamic and modelling studies at international facilities such as ISIS and the Diamond Light Source to conduct neutron and X-ray scattering experiments, and the Advanced Light Source in Berkeley U.S. to conduct single crystal diffraction work, they have been able to determine precise measurements of SO2 within MOFs at a molecular level.
Lead author of the research paper Gemma Smith said the new material shows an adsorption of SO2 higher than any other porous material known to date. This work is unprecedented as the new material is remarkably stable to SO2 exposure, even in the presence of water, and the adsorption is fully reversible at room temperature.
"Our material has been shown to be extremely stable to corrosive SO2 and can effectively separate it from humid waste gas streams. Importantly, the regeneration step is very energy-efficient compared to those reported in other studies; the captured SO2 can be released at room temperature for conversion to useful products, whilst the metal-organic framework can be reused for many more separation cycles."
More information: Reversible coordinative binding and separation of sulfur dioxide in a robust metal–organic framework with open copper sites, Nature Materials (2019). DOI: 10.1038/s41563-019-0495-0
Journal information: Nature Materials
Provided by University of Manchester