If our body temperature increases by even 1°C, we fell pretty sick, but Echinolittorina malaccana periwinkles routinely experience and survive temperatures in excess of 55°C. It turns out that the thermotolerant molluscs reinforce the core of proteins such as malate dehydrogenase, to prevent them from unraveling at high temperature, while increasing the flexibility of regions that are essential for the protein's mechanism to ensure that the protein remains functional at high and low temperatures.
No matter how warm it gets outside, most endotherms maintain a reasonably stable internal temperature; if they don't, there's usually a very good reason for their temperature to rocket. However, the body temperatures of ectothermic species that cling to life on the rocky tidal shore are not so dependable. When the tide is out and some molluscs are left high and dry on the sea shore, their body temperatures can soar to levels where the delicate protein structures within cells could begin to unravel. Yet, the remarkable Echinolittorina family of periwinkle molluscs routinely experience perilously high body temperatures in excess of 50°C as they bake in the sun. Intrigued by the extraordinary resilience of these diminutive creatures, George Somero from Stanford University, and Yun-wei Dong from Xiamen University, China, decided to find out how members of the robust family reinforce delicate protein structures so that they remain intact at temperatures where other proteins would disintegrate.
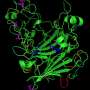
Choosing the ubiquitous cytosolic enzyme malate dehydrogenase, which produces malate for use by mitochondria in the production of ATP, Ming-ling Liao and Shu Zhang collected two members of the Echinolittorina family - E. malaccana, which can tolerate temperatures in excess of 55°C, and the less robust E. radiata - to find out what gives E. malaccana proteins the thermal edge. After isolating the enzyme from both molluscs, Liao and S. Zhang provided the enzymes with oxaloacetic acid at temperatures ranging from 20 to 40°C and measured the rate of conversion of the acid to malate, and found that E. malaccana malate dehydrogenase functioned better at high temperatures than the E. radiata enzyme. And when the duo measured the proteins' stability by holding them at temperatures up to 57.5°C and recording how their activity altered over an hour, the E. radiata malate dehydrogenase was completely inactive by the end of the experiment, whereas the more resilient E. malaccana protein was still functional, although at a much lower rate.
Curious to find out why the E. malaccana enzyme was so much tougher than the E. radiata malate dehydrogenase, Liao and S. Zhang analysed the sequence of amino acids in both proteins and identified two key locations - position 48 and 114 in the peptide chain - where the serine amino acids in the E. radiata protein were replaced by the much smaller glycine amino acids in E. malaccana malate dehydrogenase. Wondering how these subtle differences might affect the proteins, Liao, Guang-ya Zhang and Yun-meng Chu calculated how increasing heat would affect the proteins' function by effectively boiling them up to 42 and 57°C in computer in simulations that replicated the effects of different temperatures on the delicate protein structures. Analysing the calculations, the team found that the core of the E. malaccana enzyme was much more stable at 57°C than that of the E. radiata enzyme. However, the simulations also showed that regions of the more heat-resistant protein that were involved in the conversion of oxaloacetic acid into malate became more flexible. The team publishes their discovery in Journal of Experimental Biology and they suggest that these local increases in flexibility could permit the enzyme to continue functioning at lower temperatures while allowing the protein to remain stable at higher temperatures where other proteins would collapse.
More information: Liao, M., Zhang, S., Zhang, G., Chu, Y, Somero, G. N. and Dong, Y. (2017). Heat-resistant cytosolic malate dehydrogenases (cMDHs) of thermophilic intertidal snails (genus Echinolittorina): protein underpinnings of tolerance to body temperatures reaching 55°C. J. Exp. Biol. 220, 2066-2075. DOI: 10.1242/jeb.156935
Journal information: Journal of Experimental Biology
Provided by The Company of Biologists