Chicken fat, pork fat or beef fat –– none is the cornerstone of a healthful diet –– but animal fats, including those from alligators, could give an economical, ecofriendly boost to the biofuel industry, according to researchers who reported a new method for biofuel production here today. The report, following up on their earlier study on the potential use of gator fat as a source of biodiesel fuel, was part of the 247th National Meeting of the American Chemical Society, the world's largest scientific society.
The meeting, attended by thousands of scientists, features more than 10,000 reports on new advances in science and other topics. It is being held at the Dallas Convention Center and area hotels through Thursday.
"Conversion of animal fat to biodiesel has been around for some time, but the traditional biodiesel process generates significant quantities of solid waste," said Thomas Junk, Ph.D. "Our new method creates hardly any such residues." He also said that the new study concluded that using fat from such common sources as chicken, pork and beef could be much more practical for commercial implementation than from the limited amount available from alligators and could be just as effectively turned into biodiesel.
Junk, who is with the University of Louisiana at Lafayette, explained that in the earlier alligator fat study, they used a batch reactor, but switched to a flow reactor to process the fat in the new work. "We set up a flow reactor, and the reaction converting alligator fat to biodiesel happened within a few minutes," said Junk. "That's important for commercial manufacturing, where you want to produce as much fuel as quickly as possible." With batch reactors, reactions occur one-at-a-time in discrete batches. But in a flow reactor, the reactions run in a continuous stream.
He said that fuel produced from various animal fats is very similar to biodiesel manufactured by traditional, well-established methods, such as producing ethanol from corn. "So this approach is not really about a brand-new fuel, but the manufacture of a known type of fuel (biodiesel) using a more efficient, less wasteful process that largely eliminates solid-waste byproducts," he explained. This method does not require a catalyst, which creates a residue, he explained. Instead, they use "supercritical methanol," which is heated to pressures and temperatures high enough to take on properties in between those of a liquid and a gas.
Another advantage of the supercritical method is that the fat doesn't have to be extracted for the process to work, Junk said. It can be used in its raw form. Crude fat and methanol would be homogenized into a slurry (semiliquid mixture) and pumped into the system. This should be a straight-forward, simple process for a manufacturer, he added.
In the earlier study, Junk and the team noted that most of the 700 million gallons of biodiesel produced in the United States (2008 data) came from soybean oil. But there has been growing concern that using soybeans and other food crops for this purpose could raise food prices.
In searching for alternative biodiesel materials, they discovered that waste alligator fat, millions of pounds of which are thrown out every year, could also work. Their experiments showed that oil extracted from alligator fat can easily be converted into biodiesel. And now they plan to test other waste animal fats, such as those from chickens and cows. They predict that these fats also can be easily converted to biodiesel with the flow reactor system.
More information: Biodiesel from alligator fat: A comparison between supercritical and conventional transesterification conditions:
Abstract
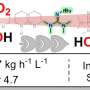
The rapid increase of alligator farming in the southeastern United States generates waste fats in large quantity. Their conversion to biodiesel by base catalyzed transesterification under traditional conditions, which involves heating of the rendered fat and methanol to reflux in the presence of sodium hydroxide as a catalyst, generates solid waste in significant quantity. Consequently, the use of a supercritical methanol as both reactant and reaction medium was investigated. This method was shown to be compatible with unprocessed (instead of rendered) fat and resulted in a near-complete conversion to liquid products. No catalyst was required, simplifying product purification. Conversion rates as a function of temperature and fat/methanol ratio were determined; conversions of up to 95.8 % of available fatty acid content to methyl esters could be achieved at 300 °C. The fate of organic nitrogen compounds (e.g., proteins) was determined.
Provided by American Chemical Society